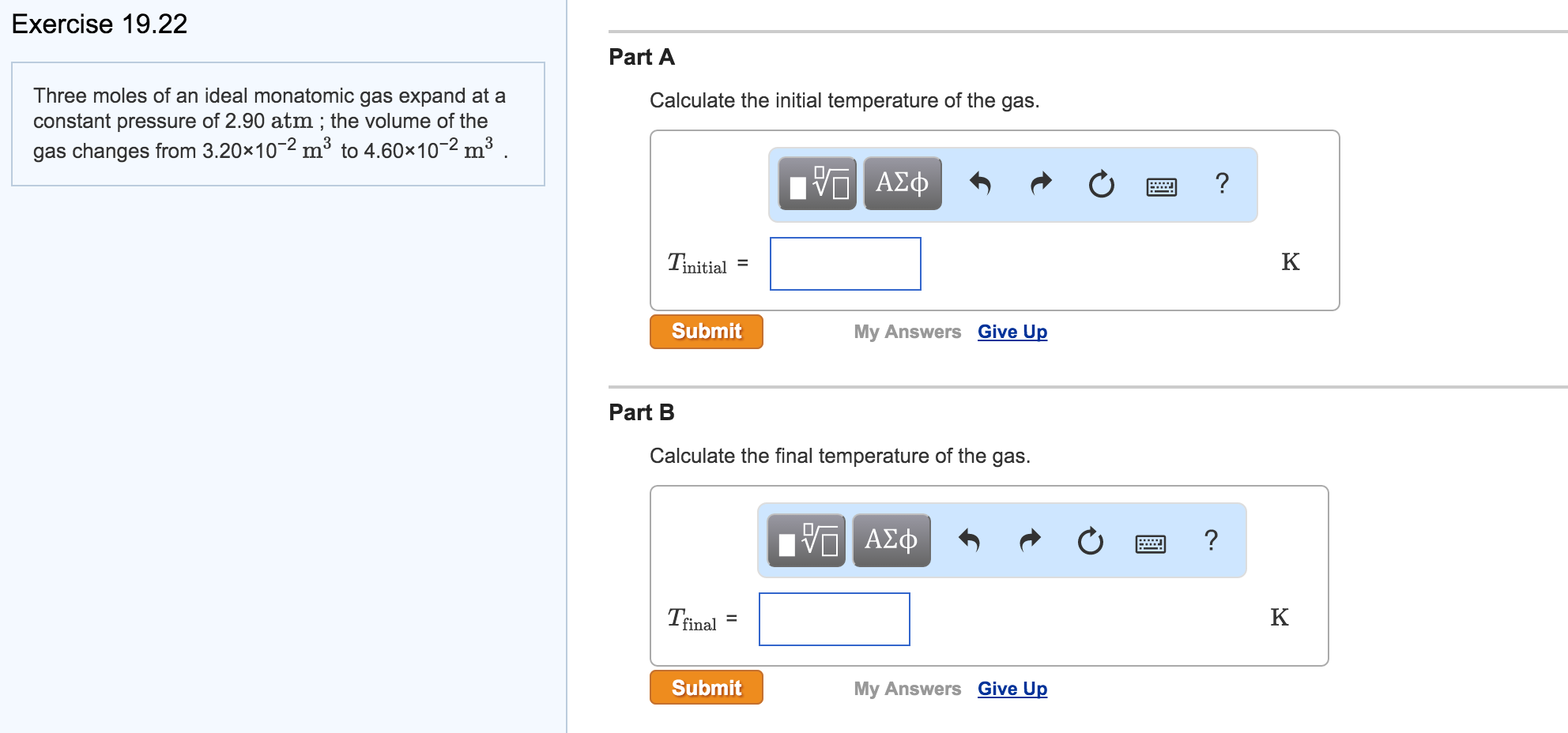
For excellent gases the coefficient of thermal growth is 1/273 at 0 levels C. A temperature boost of 1 degrees C transforms the volume of a gas by 0.366 %. At 25 degrees C the quantity of 1 g of water is 1.00296 mL, at 26 levels C the volume is 1.00323 or a change of concerning 0.026 %. London dispersion pressures occur in between atoms or molecules of nonpolar substances. Monoatomic atoms, diatomic particles and also nonpolar substances (CH4, CCl4, BF3, BeH2, and so on) are all identified by a symmetrical sharing of electrons in the atom or particle.
Oxygen intercalates slowly right into strong fullerites stored in air yet can usually be removed by pumping at raised temperature level. Nevertheless, intercalated oxygen can additionally react to create fullerene oxide.
And Also Directly Symmetrical To A Property Of Each Particle Called Polarizability.
Noble gases have numerous https://storeboard.com/blogs/general/california-medical-professionals-supplements/4532413 uses, for instance, they are utilized in neon indicators (Fig 2.16). Electrostatic forces hold atoms with each other in particles-- like both hydrogen atoms held together in H2 gas. Electrostatic pressures likewise hold electrons and protons with each other in the atom.
Are all elements Monatomic?
Some elements are monatomic, meaning they are made of a single (mon-) atom (-atomic) in their molecular form. Helium (He, see Fig. Another form of oxygen, ozone (O3), has three atoms, and sulfur (S8) has eight atoms. All elemental molecules are made of atoms of a single element.
All the halogen anions carry a -1 fee because the halogen team is one group to the left of the worthy gases in the routine chart. The oxide and sulfide ions carry a -2 charge because they lie 2 groups far from the honorable gases in the regular graph. Following this reasoning, one can anticipate that the nitride ion and the phosphide ion need to bring a -3 fee. Some of the simple anions and their names are noted in Table III. The hydride, peroxide, superoxide, and also carbide ions are exceptions to the above guideline. Atoms are small fragments found in a compound - their identity identifies the chemical element, which subsequently influences the chemical behaviour of the substance. When atoms bond with each various other, they form particles. Analogously, we can say that components incorporate to create substances.
All Of These Properties Can Be Understood In Terms Of The Intermolecular Eye-catching.
Then, name the extra electronegative component as if the extra electronegative element is a straightforward anion (finishing with-- ide). How does one understand which component is the electropositive element? If one follows this regulation, after that, SO2 would certainly be called sulfur oxide, and also Carbon Monoxide would be called carbon oxide. Really frequently, two nonmetals can integrate to form greater than one compound.
- Noble gases have a full external valence covering making them instead non-reactive varieties.
- The majority of operate in this area has actually focused on the steels lanthanum, yttrium, and scandium, which can form endohedral substances with x approximately 3.
- The only chemical elements that are steady single atom particles at conventional temperature and also pressure are the worthy gases.
- Spectroscopic experiments reveal that the metal ions donate electrons to the fullerene cages and bulk endohedral substances should thus be metallic.
Other elements include 2 or even more atoms in their molecular type (Fig. 2.8). One more form of oxygen, ozone, has 3 atoms, as well as sulfur has 8 atoms. All elemental molecules are made of atoms of a solitary aspect.
How can one identify an acid by looking at its chemical formula? You will discover the homes of acids thoroughly in the second semester of general chemistry. Below we will simply offer the regulations for naming acids.
The noble gases and mercury occur as monatomic species, whereas all various other gases as well as bromine are diatomic particles. The weakest sort of intermolecular forces are the dispersion pressures. Thermal growth is the rise in quantity of a substance with boosting temperature.